Are you looking to Hire Experienced Marketing or Tech Resources?
I am Max – a full stack developer and “I and my 40 colleagues Build Effective Web & Mobile Solutions for Startups, Small Businesses & Enterprises” My Colleague Sunny and his team help with Digital Marketing, and Deep helps with other remote resources. So we are a 1 stop solution for all your Remote hiring.




Our Work has been Featured on






What All I Offer
At Team Vipra, we specialize in building high-performing remote teams for our clients. We have a global network of talented professionals who are skilled in a variety of fields. We can help you find the right people for all your task that can be worked out remotely.

Web Development
With huge experience, weoffer front-end developers and backend developers expertise in HTML/CSS, theme development, and Javascript including, Angular, Vue, and React.
App Development
Do you have an App Idea and you are looking for a dedicated team to execute the Idea, we have covered you, with experience in a variety of niches our developers are ready to assist you.
Dedicated Tech Resources
Almost all our developers are experienced in full-stack development. With a transparent hiring process, you can now try a developer for free for 40 hours.

RPA /RDA / AI / ML
We have expertise in building complex AI and ML models. We have helped organization automate their process using RPA. With the deep knowledge on BPM we excel in this.

Digital Marketing
We have a dedicated team of marketers making Team Vipra a 1-stop solution for organizations. We have a massive experience with Search Engine Optimization and Google Adwords.
Dedicated Remote Resources
Do you have an App Idea and you are looking for a dedicated team to execute the Idea, we have covered you, with experience in a variety of niches our developers are ready to assist you.
Our Marketing Solutions
Content Marketing
Brand Visibility
Social Marketing
Search Engine Optimization
Email Marketing
PPC Advertising
Our Marketing Service
What all do we offer in Digital Marketing?
We have a team of 34 marketers with expertise in SEO, SMO, Adwords, Email Marketing, Influencer Marketing and more. We have helped several small businesseand startups grow. We have s unique strategy that helps us drive the best ROI for your marketing campaigns. Lets Talk.

Team Vipra’s Expertise
My Specialisation in Technologies
We specialize in Complete Digital solutions by offering right from Creation to Promotion, this makes us perfect for any startup or established organization. Our Experience with a variety of industries makes us competent in using various technologies and the latest tools to get the best product designed and with a team of qualified marketers, we make a perfect team for you.
Successfully completed projects
96.5%
Clients satisfaction rate
Our Technology Solutions
Uber
Like App
With the increase in demand for logistics Uber has become a case study for a lot of businesses. We have deployed over 8 Applications Using Uber Concept.
Amazon
Like App
eCommerce store has been the new shopping destination and thus small businesses are looking at buying and selling platforms giving an opportunity to create marketplaces.
Food Delivery
App
With the rise in online food ordering trend, food delivery app has seen a massive rise in buyers. This creates an opportunity for entrepreneurs.
WhatsApp
like App
Communication application has its own benefit, it’s no longer just a communication app. Entrepreneurs have started using it as a part of their core service area.
We have been Assessed by Top Review & Research Firms
My team has proved that we can deliver and listed below are few organisation that has awarded us after checking our credentials and verified testimonials.

Freelancer Portal
Upwork 99% Job Success Score
We have achieved 99% Job Success Score and 5* Ratings and Ranked as Top Rated Agency.

Digital Marketing
Ranked 18th in Top SEO Agencies
Based on the results from 5 of our client we have been ranked at 18th in Top 100 SEO firms.
Find Best Firms
Top WordPress Development Companies
We have been ranked in top 10 for best WordPress development firms in 2023 by Find Best Firms
portfolio
I'm not just talking. Check some of the Portfolio and Case studies.
eCommerce Website Design
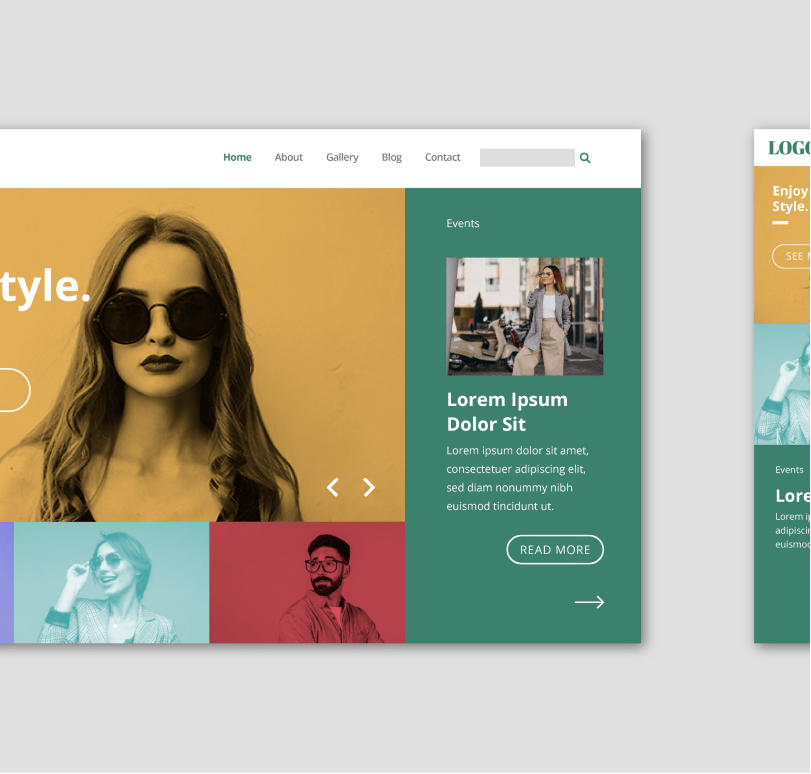
Baby Products Shopify eCommerce
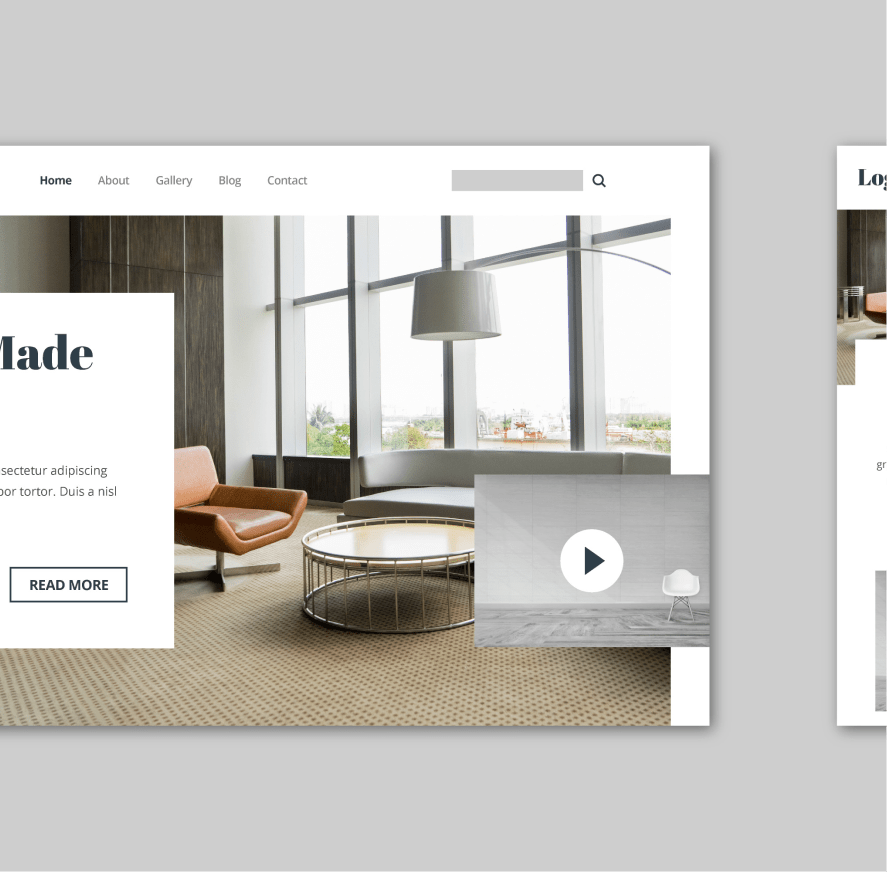
Interior designer website
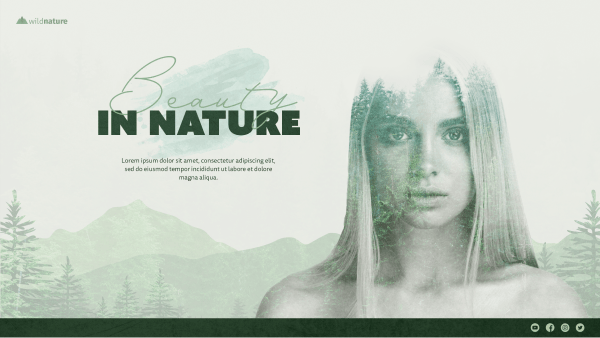
Green Tea eCommerce Complete Marketing
Cosmetics store website
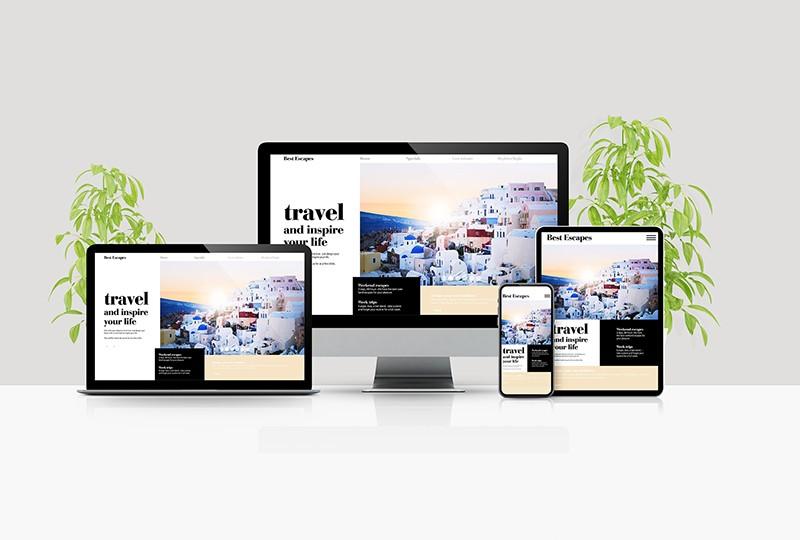
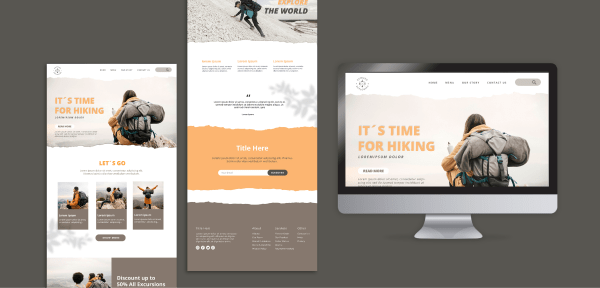
Traveling Website
Testimonial
Milk Snob
WMS.org
Trusted By Clients
At Vipra, we don’t just build brands, we build trust. For over [years of experience], we’ve been the agency clients turn to for their most critical marketing needs. Why? Because we deliver. Our award-winning team is an extension of your own, collaborating to craft data-driven strategies that resonate. We’re transparent, proactive, and passionate about exceeding expectations. From Large companies to small startups, clients of all sizes choose Vipra.







Partners & Affiliations






Our Working Process
We are an Agile team following the process that helps clients to choose what they want. Keeping it transparent we ensure you enjoy the process of onboarding.

Requirement Gathering
A Detailed interview of your business idea, automation process, Goals, vision, Competitors, and more helps us document the requirement.
Effort Calculation
Based on the requirement we create an effort breakdown module-wise of the entire project. This helps us determine resource planning and budget planning.


Project Initiate
We are an Agile team and as per the process, once the project is approved, developers are assigned using a PMS, and module-wise delivery is planned.

Research & Concept
We’re a team of non-cynics who truly for work and for each other.
Engagement Models
A relation tailored to your needs. From an individual developer to a dedicated project team, we have all you need. Our three effective partnership models are designed to adapt to individual business requirements and multiply profits for our clients.

Fixed Cost Projects
Based on your vision for the project we prepare a detailed Functional Related Document in which we elaborate on module-wise breakdown and detailed effort hours hence giving you a fixed cost.
- Best for Small Business Owners
- Best for people who would like to try SEO.
- Best for business who are on budget.

Hourly Short Term
This is Time and Material model, whenever you have a fixed requirement that you can define you can hire a developer for the short term and
- Best for teams looking for specific expertise.
- Hire when required for specific hours.
- No Long Term commitment required.

Dedicated Marketing Team
For teams who want to be very aggressive in marketing and have huge Goals to achieve. Hire teams with fewer duration commitments.
- Best for Large business with high budgets
- Have full time growth marketing team.
- Hire in just a matter of few hours.
Want to get help?
Looking to get assistance be it marketing or development, call our experts and we can get you a professional as per your requirement.
RIsk Free Contracts
All plans start with 40 Hours of free trials. If you like it we continue else we replace or end the contract, no questions asked no payments are required. 100% Risk-Free
Verified Experience
All Developers come with verified experience in development. All in-house developers with internal NDA contracts and full background checks through 3rd party agencies, so you get the best without investing in the verification part.

Quick Turn Around time
Now you can hire the developers right within 48 hours max. We have a buffer of almost 25% on maximum time on almost all the stacks. We have tie-ups with over 10 recruitment agencies and we keep on hiring.
Flexible Engagement Options
With a variety of engagement models, you can now hire a dedicated resource and side by side hire developers on time and material model, so be it a package of full time and part-time, it always as per your requirements.
Let’s Discuss
Contact Us
Call Us
+1 (843) 594-0560
+91 85869 29882



